 |
By Jamshed Arslan Pharm.D.
von Hippel-Lindau (VHL) disease is associated with tumors arising in multiple organs. Activation of hypoxia-inducible factor (HIF)-alpha underlies the VHL disease pathogenesis. In normoxia, VHL tumor-suppressor protein (pVHL) and E3 ubiquitin ligase lead to proteosomal degradation of HIF-alpha. In hypoxia, HIF-alpha escapes degradation, partly because pVHL binding to HIF-alpha depends on a posttranslational modification (hydroxylation of proline residues) on HIF-alpha that only occurs in normoxia. The exact role of pVHL in tumor hypoxia, when HIF-alpha is stabilized, is poorly understood. A multinational team of researchers has recently shown that HIF-alpha stability results from the displacement of E3 ligase when pVHL physically interacts with a metabolic enzyme, fatty acid synthase (FASN).
The team’s quest for novel regulators of pVHL started with affinity purification and mass spectrometry of the proteins associated with pVHL in colorectal HCT116 cancer cells. Silver staining revealed over a dozen bands on SDS polyacrylamide gel, but co-immunoprecipitation assays singled out FASN as a major protein that physically interacts with pVHL. To understand the functional importance of this interaction, FASN was knocked down via siRNA, which downregulated HIF-1 alpha and HIF-2 alpha in both normoxia and hypoxia. Genome editing using CRISPR-Cas9 system to knockout FASN showed similar results, but the downregulation of several HIF-alpha-target genes occurred without significant changes in mRNA levels of HIF-1alpha or HIF-2 alpha. This indicated that FASN positively regulates HIF-alpha protein levels post-transcriptionally.
Interestingly, pVHL-deficient cells did not exhibit any regulation of HIF-alpha by FASN, implying that it is the interaction with pVHL that enables FASN to regulate HIF-alpha. To highlight the underlying molecular mechanism behind this phenomenon, pull down assays were performed. The results showed that physical interaction between pVHL and FASN displaces E3 ligase components to regulate HIF-alpha.
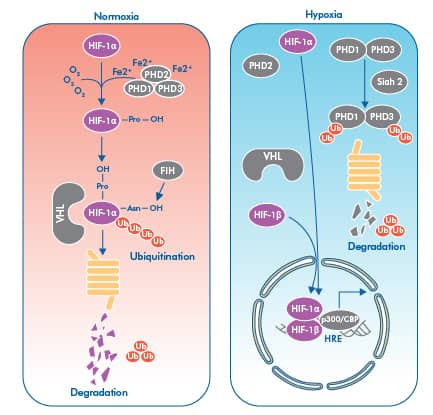 Key steps in the regulation of HIF-1 alpha. Under normoxic conditions, HIF-1 alpha is targeted for hydroxylation by a group of PHD enzymes, ensuring together with pVHL the proteasomal targeting of HIF-1 alpha. Under hypoxia HIF-1 alpha is stabilized and freely moves to the nucleus where it forms a heterodimer with HIF-1 beta and by interacting with co-activators p300/CBP regulates the expression of HRE positive target genes.
Key steps in the regulation of HIF-1 alpha. Under normoxic conditions, HIF-1 alpha is targeted for hydroxylation by a group of PHD enzymes, ensuring together with pVHL the proteasomal targeting of HIF-1 alpha. Under hypoxia HIF-1 alpha is stabilized and freely moves to the nucleus where it forms a heterodimer with HIF-1 beta and by interacting with co-activators p300/CBP regulates the expression of HRE positive target genes.
Since FASN expression is driven mainly by sterol regulatory element-binding protein 1 (SREBP1), the researchers used an SREBP1-inhibitor, called 25-hydroxycholesterol (25-OH). This natural cholesterol metabolite decreased the expression of FASN, HIF-1 alpha, and HIF-2 alpha. Injecting mice with 25-OH also reduced the mRNA expression of Fasn and Vegfa (a HIF-target gene) in their livers.
Furthermore, the team used mouse 3T3-L1 cells which undergo pre-adipose to adipose conversion upon administration of certain inducers. During this conversion, Fasn expression is also induced. Elevated protein levels of HIF-1 alpha, HIF-2 alpha, and Redd1 (a protein from a HIF-target gene), and higher mRNA expression of HIF-target genes (Vegfa, Pgk1 and Pdk1), in correlation with increased Fasn levels, were found in the differentiating 3T3-L1 cells. Along these lines, a positive correlation between FASN and HIF-target gene products (VEGFA, REDD1, ADM, KDM3A, PKM2, PGK1, HK2, and PDK1) was found in prostate and gastric cancer samples. These findings show constitutive co-overexpression of FASN and HIF-target genes as a plausible oncogenic mechanism.
This study has revealed an unexpected link between HIF and lipid metabolism (through FASN) in cancers where an oxygen-independent cellular hypoxic response can be activated. The team has shown that it is the co-expression of FASN and HIF-alpha that maintains a malignant state. Understanding these oncogenic mechanisms will pave the way for therapeutic applications targeting FASN-pVHL interactions, which is a key pathway in many cancerous conditions.
Read New White Paper on Cellular Adaptations to Hypoxia
 Jamshed Arslan, Pharm D.
Jamshed Arslan, Pharm D.
University of Alabama at Birmingham, School of Medicine
Dr. Arslan studies cell signaling in mitochondrial defects in C. elegans and transgenic mice.
References
Sun et al. “Interaction between von Hippel-Lindau Protein and Fatty Acid Synthase Modulates Hypoxia Target Gene Expression.” Scientific Reports, vol. 7, 2017, n.pag. doi:10.1038/s41598-017-05685-3