 |
By Jamshed Arslan, Pharm. D., PhD.
Cancers in the brain often come from tumors elsewhere in the body. Several adaptive mechanisms influence brain metastasis, such as blood brain barrier leakage that can be induced by stroma or disseminated tumor cells. The brain tumor microenvironment is complex, but the metastasizing cancer cells share common molecular features. To define such molecular characteristics of brain metastasis in vivo, a team led by Dr. Don Nguyen of Yale University School of Medicine developed an advanced RNA sequence-based approach. Their novel method identifies transcriptomic signatures shared by various metastasized tumors and distinguishes between tumor and stromal transcriptomes. Researchers transferred human tumor cells to immunosuppressed mice to generate a xenograft model of metastasis. Their innovative technique, called brain metastasis xenograft-RNA sequencing (BMX-seq), enabled them to appreciate both the tumor plasticity, e.g. altered expression of thousands of genes, and the stromal neuroinflammatory response, e.g. increased microglial-bound Tim3.
The team developed a xenograft model of metastasis by injecting human lung cancer cells with bioluminescent reporter into the immunosuppressed mice. As expected, the cancer metastasized to different parts of the brain. Their sophisticated RNA sequencing system for xenograft tissues revealed a distinctive gene expression of mouse cells and human cancer cells even at early stages of metastasis. The murine stroma had activated innate immune responses and downregulated ubiquitin pathways compared to xenograft brain metastasis. Transcriptomic analysis further revealed that tumor cells in the brain had activated pathways related to axonal guidance, epithelial-to-mesenchymal transition and calcium signaling relative to the same cells grown in culture. The team found a reduced expression of genes involved in hypoxia and angiogenesis in the metastasized cells in the brain relative to the same cells in subcutaneous tumors.
In other words, the BMX-seq technique showed that brain metastasis has transcriptomic features that are context-dependent. The next step was to study the response of brain metastatic stroma.
In the human lung cancer cells that colonized mice brain, researchers found enhanced expression of genes abundant in the central nervous system, such as AKAP5, EFNB3, CHL1, L1CAM, and NCAM1. In response to such adaptations of metastasized cells to the brain microenvironment, brain stroma exhibited a neuroinflammatory response. For example, tumor-bearing stroma had increased expression of Tim-3 and other genes related to pro-inflammatory molecules (Il1A, Il1b), tumor-associated macrophages (C1qb), activated astrocytes (GFAP), macrophage phagocytosis (CD68), oxidative stress (Ncf1, Cyba), and tissue remodeling (Ccl8, Tgf beta1).
|
 |
|
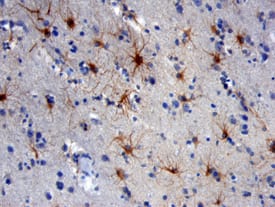
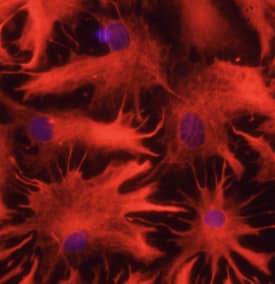
| NCAM-1/CD56 was detected in immersion fixed paraffin-embedded sections of human brain using Mouse Anti-Human NCAM-1/CD56 Monoclonal Antibody (Catalog # MAB24081) at 25 µg/mL over-night at 4 °C. Tissue was stained using the Anti-Mouse HRP-DAB Cell & Tissue Staining Kit (brown; Catalog # CTS002) and counterstained with hematoxylin (blue). View our protocol for Chromogenic IHC Staining of Paraffin-embedded Tissue Sections. | GFAP was detected in immersion fixed rat astrocytes using 10 µg/mL Sheep Anti-Human GFAP Antigen Affinity-purified Polyclonal Antibody (Catalog # AF2594) for 3 hours at room temperature. Cells were stained with the NorthernLights™ 557-conjugated Anti-Sheep IgG Secondary Antibody (red; Catalog # NL010) and counterstained with DAPI (blue). View our protocol for Fluorescent ICC Staining of Cells on Coverslips. |
In summary, the in vivo transcriptomic signature identified by BMX-seq provides a detailed map of tumor-stromal interactions during the course of brain metastasis.
The transcriptomic hallmarks provided in this research may help identify novel biomarkers of brain cancers. The RNA-based approach can improve diagnosis, and the targets identified in this paper can lead to the treatment of advanced brain tumors.
 Jamshed Arslan, Pharm D., PhD.
Jamshed Arslan, Pharm D., PhD.
Previously at the University of Alabama at Birmingham, School of Medicine.
Dr. Arslan studies cell signaling in mitochondrial defects in
C. elegans and transgenic mice
References
Wingrove, Emily, et al. "Transcriptomic Hallmarks of Tumor Plasticity and Stromal Interactions in Brain Metastasis." Cell Reports, vol. 27, no. 4, 2019, pp. 1277–1292. https://doi.org/10.1016/j.celrep.2019.03.085