Caspases, or cysteine-dependent aspartate specific proteases, are a family of enzymes crucial for initiating and executing apoptosis within a cell, an important biological event especially during organ development (1). Environmental cues and cellular signals trigger the initiation of the programmed cell death cascade primarily through proteolytic activation of the caspases (1). One specific effector caspase is caspase-3, a protein that is cleaved and thus activated upon the initiation of apoptosis. Cleaved caspase-3 propagates an apoptotic signal through enzymatic activity on downstream targets, including poly ADP ribose polymerase (PARP) and other substrates (2). In cell biology, caspase-3 antibodies that detect both uncleaved and cleaved versions of the enzyme are strong indicators of cell death induction. For example, caspase-3 antibodies are widely used to evaluate the cytotoxic strength and effectiveness of potential therapeutic agents. One study investigating the molecular mechanisms of taxane-induced apoptosis showed with caspase-3 antibodies in western blots that uncleaved caspase-3 levels decreased dramatically while activated forms of caspase-3 increased when human breast cancer cells were treated with paclitaxel (2).

Western blot analysis of whole cell protein from Jurkat cells treated with and without 2 μM staurosporine, a cell death inducing agent. The blot was probed Mouse anti-Caspase-3 (31A1067) (Pro and Active) Monoclonal Antibody (Catalog # NB100-56708), and detected with an anti-mouse HRP secondary antibody. The antibody detects both pro-caspase 3 at 35 kDa and the cleaved active caspase 3 at 15-17 kDa, and shows the active form increases expression with treatment time.
While caspase-3 canonically serves as a marker of programmed cell death, recent evidence implicates this protease as a key molecule in the pathogenesis of different cancers and neurodegenerative diseases. Specifically, levels of cleaved caspase-3 have been shown to correlate with cancer progression in tumor specimens (3). In one study, researchers demonstrated using immunohistochemistry with caspase-3 antibodies that increased levels of cleaved caspase-3 significantly associated with a higher rate of cancer recurrence and decreased survival time in cancer patients (3). Another research group at the University of Pittsburgh School of Medicine found that loss of caspase-3 sensitized cancer cells to DNA-damaging therapeutic agents (4). Researchers generated caspase-3-null colon cancer cells and revealed using immunofluorescence with caspase-3 antibodies that cancer cells lacking this enzyme were prone to undergoing necrotic events upon treatment with agents such as 5-fluorouracil (4). Finally, caspase-3 has been shown to play a role in the potentiation of Alzheimer’s Disease (AD), a disease characterized by the accumulation of amyloid-beta plaques in the brain that lead to neuronal death (5). One study from Massachusetts General Hospital demonstrated using caspase-3 antibodies that caspase-3 activation promotes the accumulation of the pathogenic neuronal proteins involved in the progression of AD (5). Together these findings indicate that caspase-3 antibodies may serve a clinical role in determining disease prognosis and progression, in addition to their more common use in identifying apoptotic cells.
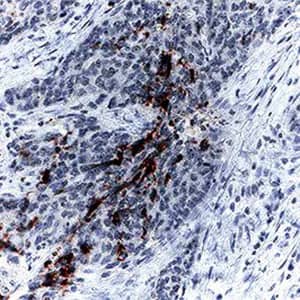
Immunohistochemical analysis of Caspase‑3 detected in human colon cancer tissue sections using Rabbit Anti-Human/Mouse Cleaved Caspase‑3 (Asp175) Monoclonal Antibody (Catalog # MAB835), followed by incubation with Anti-Rabbit IgG VisUCyte™ HRP Polymer Antibody (Catalog # VC003). Before primary antibody application, the tissue was subjected to heat-induced epitope retrieval using Antigen Retrieval Reagent-Basic (R&D Systems Catalog # CTS013). Tissue was the stained with DAB (brown) and counterstained with hematoxylin (blue). Specific Caspase-3 staining was localized to cytoplasm.
Novus Biologicals offers Caspase-3 reagents for your research needs including:
PMIDs
Note: Some headings, titles, links, and images in the blog were updated in January 2023