 |
By Beth Melson, MS
Viral infection induces the production of reactive oxygen species (ROS) by inflammatory cells, the majority of which is produced by the NOX2 oxidase. While NOX2 oxidase contributes to the clearance of bacteria and fungi, its role in viral infection is less clear, and in fact seems to contribute to increased viral pathogenicity. Recently, it was demonstrated that viruses, including influenza A, induce NOX2 oxidase production through suppression of TLR7 sensing single-stranded RNA (ssRNA)1. Therefore, inhibition of the NOX2 oxidase pathway represents an opportunity for viral suppression.
Several strains of seasonal and pandemic influenza A are capable of specifically stimulating NOX2 oxidase activity. When influenza A infects cells, NOX2 oxidase co-localizes with influenza A-containing early endosomes. Influenza A stimulates the endosomal TLR7, which senses ssRNA, to activate PKC, leading to increased NOX2 oxidase activity1. The researchers confirmed that activation of TLR7 promotes endosomal ROS production with the specific TLR7 agonist imiquimod. Viruses generally use this pathway; for example, exposure of macrophages to other viruses (i.e. rhinovirus, HIV, dengue, respiratory syncytial virus) also led to TLR7-dependent endosomal ROS production, which is distinct from bacteria-induced ROS.
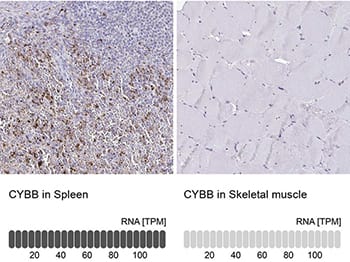 Orthogonal Strategies Validation. Immunohistochemistry-Paraffin: CYBB/NOX2 Antibody [NBP2-38642] - Staining in human spleen and skeletal muscle tissues using anti-CYBB antibody. Corresponding CYBB RNA-seq data are presented for the same tissues.
Orthogonal Strategies Validation. Immunohistochemistry-Paraffin: CYBB/NOX2 Antibody [NBP2-38642] - Staining in human spleen and skeletal muscle tissues using anti-CYBB antibody. Corresponding CYBB RNA-seq data are presented for the same tissues.
The activation of NOX2 oxidase by influenza A is beneficial to the virus as it suppresses the production of cytokines and antibodies that are necessary for viral clearance and the induction of immune memory. The hydrogen peroxide produced by NOX2 in response to influenza A infection leads to the modification of a unique cysteine residue (Cys98) on the endosomal face of TLR7, causing a reduced cytokine response to viral infection. Thus, Cys98 of TLR7 acts as a redox sensor. The researchers developed a drug that specifically inhibits endosomal NOX2 oxidase and reduces pathogenicity of influenza A, leading to better outcomes in a mouse model of infection1.
Learn more with TLRs Interactive Pathway
Influenza A causes significant morbidity and mortality worldwide2. To et al. have identified a potential method to specifically target endosomal NOX2 oxidase to suppress ROS and combat viruses1. This inhibitor could be applicable as an antiviral therapy for several viruses that activate endosomal ROS, including rhinovirus, dengue, and HIV. The authors note the importance of balancing autoimmune responses versus viral clearance, and suggest short-term suppression of endosomal ROS to limit viral pathogenicity, and thus promote clearance1.
 Beth Melson, MS
Beth Melson, MS
PhD Candidate
University of Virginia
Beth is working on her PhD studying virulence mechanisms of pathogenic E. coli.
References