 |
By Jamshed Arslan, Pharm. D., PhD.
Atherosclerosis is a chronic inflammatory condition in which plaques of fats and other substances slowly buildup on the inner walls of arteries to restrict blood flow. In atherosclerosis-associated ailments like obesity, hypertension and type II diabetes, intercellular tight junctions are impaired, leading to increased gut permeability. This means that products of intestinal microbiota like lipopolysaccharide from Gram-negative bacteria can seep into the blood stream.
A pro-atherogenic role of lipopolysaccharide has been suggested. For example, genetically deleting toll-like receptor 4 (TLR4), a lipopolysaccharide receptor, produced smaller atherosclerotic plaques in a mouse model1 and injecting lipopolysaccharide increased plaque formation in hypercholesterolemic rabbits2. However, studies on humans are lacking. To investigate if gut-derived lipopolysaccharide localizes in human plaques and to study its role in atherosclerosis in humans, a research team in Italy studied lipopolysaccharide and TLR4 in thyroid and carotid arteries from human samples3. Carotid plaques were found to be positive for lipopolysaccharide and TLR4, and this positivity/reactivity increased in areas with activated macrophages. Incubating human-derived monocytes with lipopolysaccharide showed TLR4-mediated upregulation of NOX2 – an enzyme that generates reactive oxygen species (ROS) – in a dose-dependent manner. This indicates TLR4-mediated oxidative stress in atherosclerosis by gut-derived lipopolysaccharide .
In this cross-sectional study[3], patients with abnormal narrowing of carotid artery undergoing plaque-removal surgery were compared with controls. Researchers observed higher gut permeability in patients (indicated by higher serum zonulin/haptoglobin, a regulator of gut’s intercellular tight junctions) that correlated with increased circulating lipopolysaccharide. Immunohistochemical analysis showed a pronounced atherosclerotic damage in all carotid arteries, but not in thyroid arteries. Likewise, immunoreactivity against lipopolysaccharide, TLR4 and CD68 was observed in carotid plaques, but not in thyroid arteries. They found that higher reactivity for lipopolysaccharide and TLR4 in a carotid plaque area directly correlated with the size of CD68-positive, activated macrophages.
These data show localization of lipopolysaccharide in atherosclerotic plaques with macrophage infiltration, possibly because macrophage surface has TLR4 as lipopolysaccharide receptors. The next question was: can lipopolysaccharide, in concentrations found in human atherosclerotic plaques, activate monocytes?
|
|
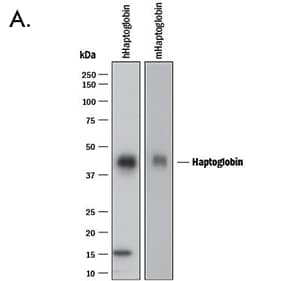
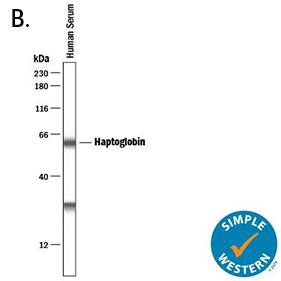
| A. Western blot shows lysates of human Haptoglobin purified from human serum and mouse haptoglobin purified from mouse serum. PVDF membrane was probed with 0.2 µg/mL of Goat Anti-Human/Mouse Haptoglobin Antigen Affinity-purified Polyclonal Antibody (Catalog # AF8465) followed by HRP-conjugated Anti-Goat IgG Secondary Antibody (Catalog # HAF017). A specific band was detected for Haptoglobin at approximately 45 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 1. | B. Simple Western lane view shows human serum, loaded at 1:25000 dilution. A specific band was detected for Haptoglobin at approximately 60 kDa (as indicated) using 1 µg/mL of Goat Anti-Human/Mouse Haptoglobin Antigen Affinity-purified Polyclonal Antibody (Catalog # AF8465) followed by 1:50 dilution of HRP-conjugated Anti-Goat IgG Secondary Antibody (Catalog # HAF019). This experiment was conducted under reducing conditions and using the 12-230 kDa separation system. |
The researchers incubated monocytes from patients and controls with biologically relevant concentrations of E. coli-derived lipopolysaccharide. They found a significant increase in oxidant species (H2O2 and conjugated dienes) in monocytes derived from patients. To determine the mechanism behind this phenomenon, they measured the levels of soluble NOX2-derived peptide (sNOX2-dp) released into the medium by lipopolysaccharide-incubated cells. Lipopolysaccharide dose-dependently increased sNOX2-dp, indicating NOX2 upregulation. This effect was abolished upon incubating cells with a TLR4-inhibitor. Similarly, incubating cells with NOX2 specific inhibitor stopped the formation of oxidative stress-related products (H2O2, conjugated dienes, oxidized LDL).
Due to the central role of NOX2 in generating ROS and killing bacteria as part of innate immune system, these findings imply that gut-derived lipopolysaccharide promotes oxidative stress at atherosclerotic lesions through a TLR4-mediated mechanism.
This is the first study to provide data on human plaques in the context of gut-derived lipopolysaccharide. Lower serum lipopolysaccharide might be an indicator of gut intactness and diminish atherosclerotic process. Since Mediterranean diet is implicated in reducing serum lipopolysaccharide4, 5, this study3 may offer nutritional advice to curb atherosclerotic progression.
 Jamshed Arslan, Pharm D., PhD.
Jamshed Arslan, Pharm D., PhD.
University of Alabama at Birmingham, School of Medicine
Dr. Arslan studies cell signaling in mitochondrial defects in
C. elegans and transgenic mice.
References